Explore the newest medical treatments to relieve the symptoms of dry eye
Dry Eye Specialist – Dr. James Lewis
Welcome to the website of Dr. James Lewis, a leading provider of eye care and dry eye treatment in New Jersey and Philadelphia, PA. Dr. Lewis has been practicing ophthalmology for more than 30 years and is proud to help his patients enhance their quality of life by relieving them of severe dry eye symptoms. Learn more about dry eye syndrome and the treatment options available below, and contact Dr. Lewis at 215-886-9090 to schedule an appointment.
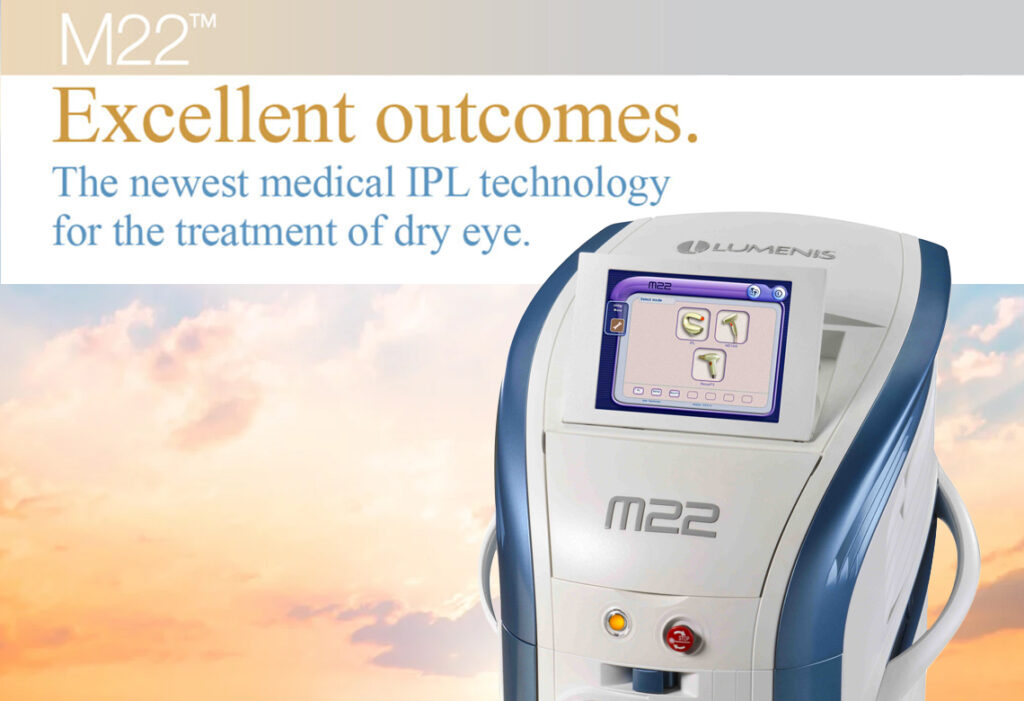
James S. Lewis, MD, a pioneer in the use of Intense Pulsed Light (IPL) for treatment of dry eye in the Philadelphia area is now the first practice to receive the Lipiflow System. Advanced IPL: Lumenis M22 is now available to those patients with meibomian gland dysfunction and evaporative dry eye. Dr. Lewis is a cornea specialist in Philadelphia and director of the Cornea and Refractive Surgery Department at the Pennsylvania College of Optometry of Salus University in Philadelphia, PA. Dr. Lewis was an early adopter of temporary and Thermal permanent punctal occlusion for the treatment of Aqueous Deficiency dry eye over 20 years ago. Evaporative Dry Eye is now the focus of our efforts to aggressively manage this widespread debilitating condition.
- Total for both eyes
- Duration: 10 minutes
- Advanced IPL: Lumenis M22
- Total for both eyes
- One MIBO treatment
- (regularly $300)
Schedule an Evaluation
Click to Call